Blog
We are excited to inform you that we have extended our environmental exposure testing to include 3 new panels. This means we are now testing for more substances for exposure. View our panels here!
Our testing will now include detection of:
- Fentanyl
- Meperidine
- Tramadol
If you have questions you can contact your account manager or speak to one of our representatives by calling 800.235.2367 or by emailing us here.
To learn more, watch our educational ToxTime event Environmental Exposure Drug Testing 101 ChildGuard.

Newborn Toxicology – A Review and Recent Developments
Please join us for ToxTime with Joseph Jones, Ph.D., NRCC-TC, Chief Operating Officer at USDTL, as he discusses the evolution of drug testing in various newborn specimen types, followed by a brief Q&A.
What You’ll Learn
- The pros and cons of newborn specimen types
- The evolution of newborn testing from urine to meconium to umbilical cord tissue
- A brief overview of previous studies
- And more…
Click Here to Watch the Webinar.
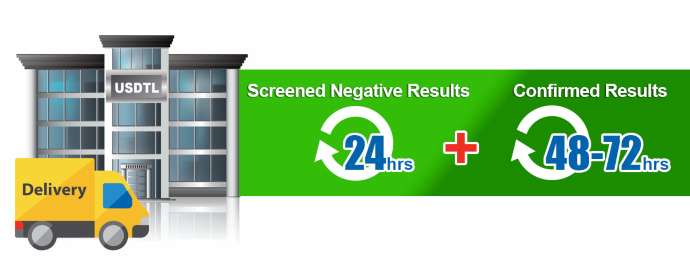
As an accredited laboratory, we have an extensive list of criteria that must be met to ensure each specimen result is forensically defensible evidence. Given the accreditation guidelines, reanalysis of evidence may be needed on specimens, or specimen batches, for various reasons. Though we try our best to avoid these situations, we have protocol(s) we must follow when this happens. Unfortunately, reanalysis can shift the anticipated turnaround time for specimen results. When it is required, we ask for your understanding and patience while further testing is being performed.

The Detection of Fentanyl in Hair Specimens
Joseph Jones, Ph.D., NRCC-TC, Chief Operating Officer at USDTL discusses the toxicology of fentanyl in hair specimens followed by a brief Q&A.
As innovators in substance abuse toxicology, USDTL has been testing for fentanyl in hair specimens for over a decade, well before it was a mainstream drug of abuse. In this webinar, we share with you what we have learned about the dangers of fentanyl specifically, and the trends we have seen to help you understand why testing in hair specimens is beneficial.
What You’ll Learn
- Why fentanyl is so dangerous compared to other opioids
- Relevant data and case report overview
- Fentanyl trends in hair specimens at USDTL
- And more…
Click Here to Watch the Webinar.

Detecting Illicit Fentanyl in Newborns
Joseph Jones, Ph.D., NRCC-TC, Chief Operating Officer at USDTL discusses the toxicology of fentanyl in newborn umbilical cord tissue followed by a brief Q&A.
What You’ll Learn
- The advantages of using umbilical cord tissue for newborn toxicology.
- Does hospital-administered fentanyl show as positive at the 500 pg/g cutoff?
- What trends are we seeing with fentanyl in umbilical cord at USDTL?
- And more…
If you sign in and attend live, you will receive a Certificate of Attendance following the Webinar!
A recording will be made available for all registrants.
Click Here for the On-Demand Link to the Webinar.
Understanding Toxicology Reports: Confirmations, Cutoffs, and the Quantitative Values
Joseph Jones, Ph.D., NRCC-TC, Chief Operating Officer at USDTL discusses confirmations, cutoffs, and quantitative values followed by a brief Q&A.
What You’ll Learn
You’ll get answers to the following questions:
- Why is Confirmation Testing so Important?
- Qualitative vs Quantitative: What does it mean?
- What Are Cutoff Levels and Why do we Have Them?
- What Does the Quantitative Result on my Report Mean?
- And more…
Click Here for the On-Demand Link to the Webinar.

By Freepik© Studio
Over the past year, Fentanyl related overdose deaths have spiked amid the pandemic. Researchers have noticed the growing spread of street drugs laced with deadly synthetic opioids including fentanyl.
Dr. Nora Volkow, head of the National Institute of Drug Abuse, said people are often consuming fentanyl “unbeknownst to them,” resulting in a spike of overdose deaths. Most people are taking fentanyl unknowingly as it’s mixed with other drugs. Drug traffickers are mixing fentanyl with other drugs, including heroin, cocaine, methamphetamine, and MDMA, because it takes little to produce a high, making it a cheap option.
Riverside County District Attorney, Mike Hestrin, says fentanyl is “now in everything”. Hestrin explained that drug traffickers are using pill-making machines to create counterfeit drugs that look like prescription medications but actually contain fentanyl. He added that drug traffickers don’t have the sophisticated technology to accurately dose pills with non-lethal quantities of fentanyl, essentially leading to a game of Russian roulette.
The DEA explains on their website that unless a drug is prescribed by a licensed medical professional and dispensed by a legitimate pharmacy, you can’t know if it’s fake or legitimate. This leaves the user with the risk of a potentially lethal dose of fentanyl laced in their drug of choice.
References
- Fentanyl overdoses, deaths are up in Riverside County. Many don’t know they’ve taken it. (2021). https://www.desertsun.com/story/news/health/2021/07/30/fentanyl-overdoses-deaths-rise-riverside-county/8043736002/
- Facts about Fentanyl. United States Drug Enforcement Agency. (2020). https://www.dea.gov/resources/facts-about-fentanyl
USDTL hosted an online Webinar, titled ToxTime, where we discussed Newborn Alcohol Biomarkers.
The Webinar discussion included:
- What is FASD?
- The prevalence of FAS.
- The current methods available for detecting prenatal alcohol exposure.
- The importance of screening moms for alcohol.
- The importance of providers testing for alcohol, even if they do not have a direct plan of care for babies exposed to alcohol.
Click Here for the On-Demand Link to the Webinar.
- Forensic vs. Clinical Drug Testing: Why Flexibility Matters for Your Organization
- USDTL’s Integration and Partnership With CourtFact
- New Year, New Capabilities: Offering Forensic & Clinical Testing Options
- Weather Delay
- The Detection of Delta-9-tetrahydrocannabinol, Delta-8-tetrahydrocannabinol, Delta-10-tetrahydrocannabinol, and Cannabidiol in Hair Specimens
- Umbilical Cord Tissue Testing for Ketamine
- Drugs of Abuse: A DEA Resource Guide (2024)
- Beyond THC and CBD: Understanding New Cannabinoids
- February 2026 (1)
- January 2026 (3)
- October 2025 (1)
- July 2025 (3)
- May 2025 (2)
- April 2025 (2)



