Meconium Collection: Nothing More, Nothing Less
Showing: ISO/IEC 17025
What you need to know about meconium collection.
by Michelle Lach, MSIMC
Meconium is the first stool of a newborn infant. It is produced in utero and consists of materials such as epithelial cells, bile, mucous, and more. In most newborns, meconium is generally passed in the first day or so of life, has no odor, and appears as a very dark, tar-like substance. This helps distinguish meconium from the next phase of passage called transitional stool.
Transitional stool will start to have an odor and present with a more brown, green, or yellow color as the newborn starts digesting milk. When drug testing the meconium of a newborn, it is important to note this difference since only meconium is created during gestation and transitional stool is created after birth. Collection of any stool other than meconium for drug testing purposes may result in a rejected specimen.
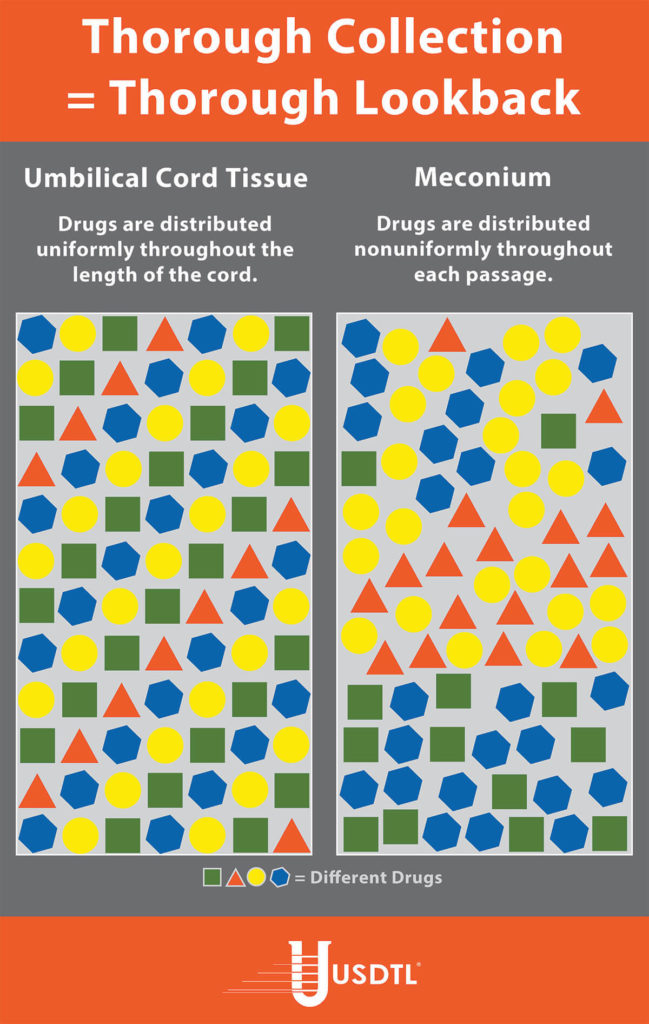
Unlike umbilical cord tissue, drugs are not distributed uniformly throughout the meconium specimen (see Figure 1). Because of this, the collection of the entire mass of meconium is highly encouraged to assure that there will be enough specimen to test, and that the maximum window of drug detection is achieved. It can take multiple passages of meconium before the newborn begins the transitional stool phase.
We require a minimum of 3 grams of meconium to be able to properly run our tests, so collecting the entire passage of meconium from newborns that have been exposed to substances of abuse is highly critical since they tend to have lower birth weights and create less specimen in the first place. If there is not enough specimen to run the test, the results are reported out as QNS. Quantity Not Sufficient (QNS) is a result of not having a sufficient quantity (volume) of specimen to test for the panels ordered.
Dear Valued Client,
We are proud to announce that we are the first laboratory in the world to be ISO/IEC 17025 accredited for drug and alcohol testing in umbilical cord, fingernail, and toenail specimens. On September 4, 2015, USDTL attained ISO/IEC 17025 accreditation showing full compliance with the international testing standards. We have received our accreditation from ANSI-ASQ National Accreditation Board, demonstrating technical competence in the field of forensic testing. The scope of our ISO/IEC 17025 accreditation encompasses all specimen types and methods of analysis utilized in our laboratory.
Our laboratory has always maintained this level of quality and competency since our humble beginnings in 1991, bringing our clients the most responsive, personal service in the drug and alcohol testing industry. ISO/IEC 17025 accreditation reaffirms that commitment to our clients, for all aspects of our testing and client advocacy. You can always have absolute confidence that the results of every specimen tested by our laboratory will meet the highest of international standards.
ISO/IEC 17025 is the single most important standard applied to testing and calibration laboratories around the globe. Laboratories accredited to the ISO/IEC 17025 standard have demonstrated that they are technically competent and able to reproducibly generate accurate, precise and consistent data.
The practical benefits for clients of USDTL of ISO/IEC 17025 accreditation are seen on a continual basis:
- Continuously produced testing results of the highest quality, validity, and integrity;
- Improved customer communication and resolution of customer issues;
- Continual improvement of our management system, with an emphasis on the responsibilities of senior management;
- Fast resolution of laboratory issues regarding methods and equipment.
- Evidential acceptance of USDTL laboratory results in virtually all jurisdictions.
To view our certificate of accreditation by ANAB 17025:2017 Forensic Science Testing and Calibration Lab. If you have questions about ISO/IEC 17025 accreditation, please contact us at clientservices@usdtl.com or 800.235.2367.
Sincerely,
Adam Negrusz, Ph.D., F-ABFT
Laboratory Director
- The Detection of Delta-9-tetrahydrocannabinol, Delta-8-tetrahydrocannabinol, Delta-10-tetrahydrocannabinol, and Cannabidiol in Hair Specimens
- Umbilical Cord Tissue Testing for Ketamine
- Drugs of Abuse: A DEA Resource Guide (2024)
- Beyond THC and CBD: Understanding New Cannabinoids
- New Xylazine, Psilocin, Gabapentin, Dextromethorphan, and Extended Cannabinoids Testing at USDTL
- Psilocin: The Magic Behind the Mushroom
- Fetal Fentanyl Syndrome: Why Detecting Newborn Fentanyl Exposure Matters Now More Than Ever
- DMT: An Overview
- October 2025 (1)
- July 2025 (3)
- May 2025 (2)
- April 2025 (2)
- March 2025 (2)
- February 2025 (1)


