Michelle Pilkington joins the Board of Directors of the Chicago Chapter of CLMA
Showing: USDTL

Congratulations to Michelle Pilkington for joining the Board of Directors for the Chicago Chapter of the Clinical Laboratory Management Association. CLMA is a community that brings together clinical laboratory professionals from laboratories of all sizes. Clinical laboratory managers and leaders, clinical technicians, consultants, marketers and military staff can connect and share their collective knowledge. To learn more click here. Michelle said “Having a seat at the table, representing USDTL, gives us an opportunity to be face to face with some of our regional clients and make a difference.”

By Adobe Stock©
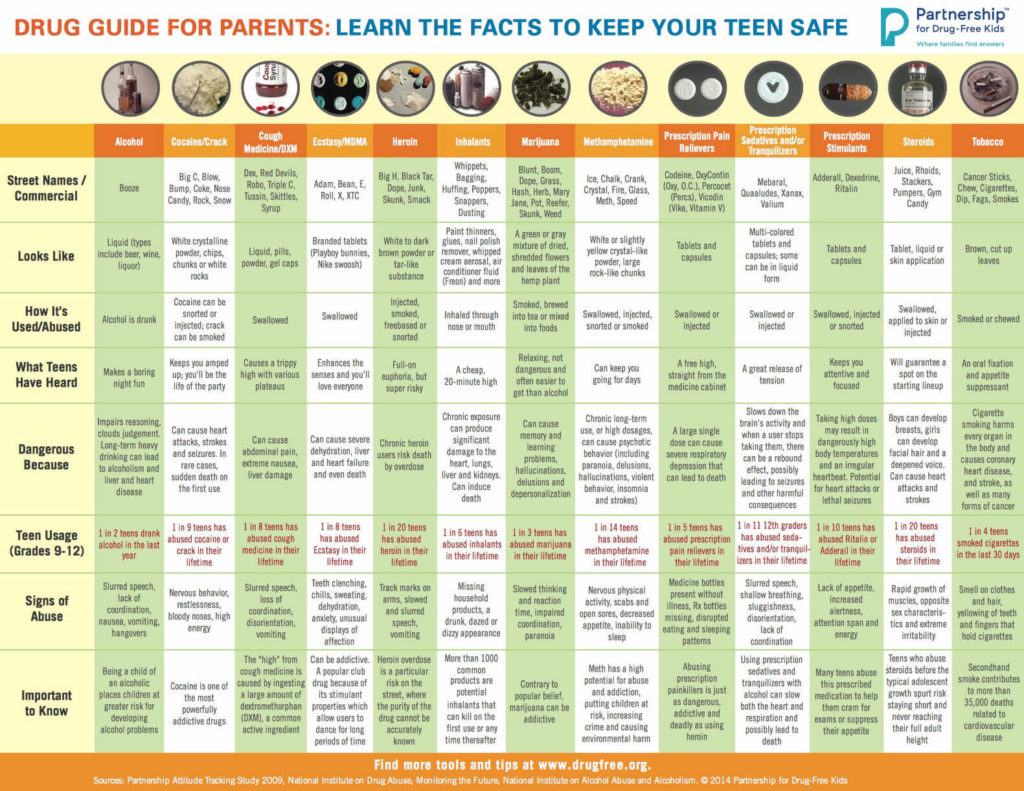
According to the Centers for Disease Control and Prevention (CDC), fentanyl-related overdose deaths in the United States have increased by more than 1,000 percent from 2011 through 2016.1 The increased availability of illicitly manufactured fentanyl has contributed to the prevalence of neonatal abstinence syndrome (NAS) or neonatal opioid withdrawal syndrome (NOWS) throughout the United States. There was a greater than five-fold increase in the proportion of babies born with NAS from 2004 to 2014, when an estimated 32,000 infants were born with NAS/NOWS —equivalent to one baby suffering from opioid withdrawal born approximately every 15 minutes.2
In response to an ongoing opioid epidemic, medical professionals and advocates are invested in providing education, resources, testing, and preventative care in efforts to combat one of the most powerful opioids in the world —Fentanyl. This synthetic, opioid is extremely addictive and when used while pregnant can cause devastating outcomes to the fetus including neural tube defects, congenital heart defects, gastroschisis, stillbirth, and pre-term delivery.3
Newborns are often diagnosed with NAS/NOWS as a direct result of a sudden interruption of fetal exposure to prescription and illicit substances that were used or abused by the mother. Diagnosing an infant with NAS/NOWS is often assessed by identifying the following symptoms: tremors, jitteriness, irritability, excessive crying, and diarrhea. These indicators have been determined in the medical field as the standards for identifying a compromised central nervous system. Prenatal use & abuse of fentanyl can increase an infant’s risk for developing NAS/NOWS.
What Makes Fentanyl So Dangerous?
According to the United States Drug Enforcement Administration (DEA), fentanyl is approximately 100 times more potent than morphine and 50 times more potent than heroin.4 Its initial development was intended as a licit intravenous anesthetic; however, it has now become a public health threat as pharmaceutical products are subjected to theft, fraudulent prescriptions and illicit or less regulated distribution and manufacturing. The drug is often abused by injecting, snorting / sniffing, oral tablets/pills or removing the gel on fentanyl patches and then injecting or ingesting. The popularity of fentanyl within drug addicted communities derive from the addictive effects the drug produces —relaxation, euphoria, pain relief, and sedation. However, abused fentanyl also produces fatal side effects including: confusion, dizziness, nausea, vomiting, urinary retention, pupillary constriction, and respiratory depression.
Fentanyl’s Long-Term Effects on Fetus
Prenatal substance abuse can lead to long-term, irreversible damage to a developing fetus. In relation to fentanyl, these effects can consist of the following when associated with NAS/NOWS:5
- Cognitive and motor development deficiencies
- Infections
- Vision complications
- Sudden Infant Death Syndrome (SIDS)
- Susceptible to future substance abuse
Fentanyl and Toxicology Confirmation
According to the American Academy of Pediatrics, NAS/NOWS is a clinical diagnosis, however toxicological confirmation is necessary to identify the exact type of substance that the mother was using or abusing and to confirm or rule out the use of other licit or illicit substances during pregnancy.6
Due to the recent increase of cases regarding pregnant women and fentanyl exposure, it is critical to utilize testing that can help detect the latest drugs of abuse. Through internal research and strategic analysis, our laboratory is combating the growing epidemic of fentanyl use among pregnant women and those of childbearing age, with the development of testing for fentanyl in both umbilical cord tissue and meconium. As the first and only laboratory to offer fentanyl testing utilizing both advanced newborn specimens, our partnerships are provided with the opportunity to proactively and effectively address the alarming rates of prenatal fentanyl use. Both meconium and umbilical cord tissue belong to the baby, therefore maternal consent is not needed to proceed in testing.
Umbilical Cord Tissue
The umbilical cord tissue is available immediately for 100 percent of births and requires only 1 collection by 1 collector. This specimen’s look back provides detection up to approximately 20 weeks prior to birth, the most advanced newborn specimen on the market.
Meconium
Meconium has been recognized for decades as the “gold standard” for the detection of substances. It is the first stool of an infant produced in utero, consisting of epithelial cells, bile, mucous, and is odorless with a very dark, tar-like appearance. Meconium is uniquely developed during gestation with exclusive capabilities of preserving substances exposed prenatally up to approximately 20 weeks prior to birth. The average start of a meconium passage after birth occurs within 24-48 hrs., in most newborns it is generally passed in the first day or so of life.
Turnaround Time
Our methodology and advanced instrumentation contribute to our laboratory’s efficient turnaround time. Generally, the standard turnaround time for reporting negative screening test results is the next business day, with an additional 1-2 business days for specimens that require confirmatory testing. Turnaround time begins from receipt of the valid specimen–accompanied by a properly documented valid order– into the laboratory. Some tests require additional time to process and will fall outside the standard turnaround time window.
As one of the only laboratories in the nation focusing exclusively on substance abuse testing, we are consistently adapting our services to meet the needs of our clients and communities. The validity of newborn toxicology results has never been more imperative. Through our partnership and services, we can customize a flexible comprehensive drug testing program based on your population health needs. Today’s substance abuse landscape is drastically different than it used to be-collaboration is vital in supporting our mission of protecting and enriching lives.
References
- Spencer, M.P.H., M., Warner, Ph.D, M., Bastian, B.S., B., Trinidad, M.P.H., M.S., J. and Hedegaard, M.D., M.S.P.H., H. (2019). “National Vital Statistics Reports.” [online] Cdc.gov. Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_03-508.pdf [Accessed 17 Sep. 2019].
- Drugabuse.gov. (2019). “Dramatic Increases in Maternal Opioid Use and Neonatal Abstinence Syndrome.” [online] Available at: https://www.drugabuse.gov/related-topics/trends-statistics/infographics/dramatic-increases-in-maternal-opioid-use-neonatal-abstinence-syndrome [Accessed 17 Sep. 2019].
- Center for Disease Control and Prevention, U. (n.d.). “Pregnancy and Opioid Pain Medications.” Retrieved from https://www.cdc.gov/drugoverdose/pdf/pregnancy_opioid_pain_factsheet-a.pdf.
- Department of Education, U. (n.d.). “Drugs of Abuse Guide” (2017)(pp. 40-41) (United States).
- “Long-Term Outcomes of Infants With Neonatal Abstinence Syndrome.” (n.d.). Retrieved from https://www.seattlechildrens.org/globalassets/documents/healthcare-professionals/neonatal-briefs/neonatal-abstinence-syndrome-outcomes.pdf
- Kocherlakota, P. (2014, August 01). “Neonatal Abstinence Syndrome.” Retrieved from http://pediatrics.aappublications.org/content/134/2/e547
To view the larger image, please click here.
What you need to know about meconium collection.
by Michelle Lach, MSIMC
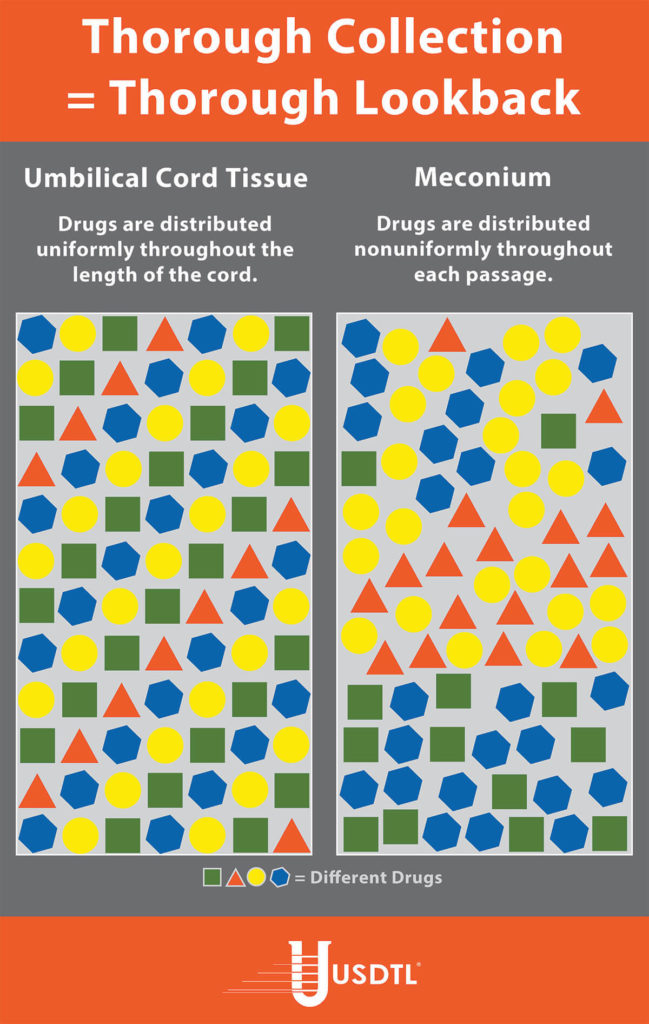
Meconium is the first stool of a newborn infant. It is produced in utero and consists of materials such as epithelial cells, bile, mucous, and more. In most newborns, meconium is generally passed in the first day or so of life, has no odor, and appears as a very dark, tar-like substance. This helps distinguish meconium from the next phase of passage called transitional stool.
Transitional stool will start to have an odor and present with a more brown, green, or yellow color as the newborn starts digesting milk. When drug testing the meconium of a newborn, it is important to note this difference since only meconium is created during gestation and transitional stool is created after birth. Collection of any stool other than meconium for drug testing purposes may result in a rejected specimen.
Unlike umbilical cord tissue, drugs are not distributed uniformly throughout the meconium specimen (see Figure 1). Because of this, the collection of the entire mass of meconium is highly encouraged to assure that there will be enough specimen to test, and that the maximum window of drug detection is achieved. It can take multiple passages of meconium before the newborn begins the transitional stool phase.
We require a minimum of 3 grams of meconium to be able to properly run our tests, so collecting the entire passage of meconium from newborns that have been exposed to substances of abuse is highly critical since they tend to have lower birth weights and create less specimen in the first place. If there is not enough specimen to run the test, the results are reported out as QNS. Quantity Not Sufficient (QNS) is a result of not having a sufficient quantity (volume) of specimen to test for the panels ordered.
Neonatal Drug Withdrawal
By Freepik© Studio
Maternal Nonnarcotic Drugs that cause neonatal psychomotor behavior consistent with withdrawal.
The Onset of Newborn Withdrawal Symptoms is Highly Variable
| Drug | Onset of Signs |
| Diazepam | Hours to Weeks |
| Alcohol | 3-12 Hours |
| Heroin | 24 Hours |
| Sedatives | 1-3 Days |
| Methadone | 1-7 Days |
| Opiates | 1-7 Days |
| Barbiturates | 1-14 Days |
– Click here to download the pdf.
Read an excerpt from the article Neonatal Drug Withdrawal below:
Signs characteristic of neonatal withdrawal have been attributed to intrauterine exposure to a variety of drugs. Other drugs cause signs in neonates because of acute toxicity. Chronic in utero exposure to a drug (eg, alcohol) can lead to permanent phenotypical and/or neurodevelopmental-behavioral abnormalities consistent with drug effect. Signs and symptoms of withdrawal worsen as drug levels decrease, whereas signs and symptoms of acute toxicity abate with drug elimination. Clinically important neonatal withdrawal most commonly results from intrauterine opioid exposure. The constellation of clinical findings associated with opioid withdrawal has been termed the neonatal abstinence syndrome (NAS). Among neonates exposed to opioids in utero, withdrawal signs will develop in 55% to 94%.1,2 Neonatal withdrawal signs have also been described in infants exposed antenatally to benzodiazepines,3,4 barbiturates,5,6 and alcohol.7,8
— Neonatal Drug Withdrawal https://doi.org/10.1542/peds.2011-3212
References:
-
Harper RG, Solish GI, Purow HM, Sang E, Panepinto WC. The effect of a methadone treatment program upon pregnant heroin addicts and their newborn infants. Pediatrics. 1974 ; 54 (3): 300–305 [PubMed]
-
Ostrea EM, Chavez CJ, Strauss ME. A study of factors that influence the severity of neonatal narcotic withdrawal. J Pediatr. 1976; 88 (4 pt 1): 642–645 [PubMed]
-
Rementería JL, Bhatt K. Withdrawal symptoms in neonates from intrauterine exposure to diazepam. J Pediatr. 1977; 90 (1): 123–126 [PubMed]
-
Athinarayanan P, Piero SH, Nigam SK, Glass L. Chloriazepoxide withdrawal in the neonate. Am J Obstet Gynecol. 1976; 124 (2): 212–213 [PubMed]
-
Bleyer WA, Marshall RE. Barbiturate withdrawal syndrome in a passively addicted infant. JAMA. 1972; 221 (2): 185–186 [PubMed]
- Desmond MM, Schwanecke RP, Wilson GS, Yasunaga S, Burgdorff I. Maternal barbiturate utilization and neonatal withdrawal symptomatology. J Pediatr. 1972; 80 (2): 190–197 [PubMed]
- Pierog S, Chandavasu O, Wexler I. Withdrawal symptoms in infants with the fetal alcohol syndrome. J Pediatr. 1977; 90 (4): 630–633 [PubMed]
- Nichols MM. Acute alcohol withdrawal syndrome in a newborn. Am J Dis Child. 1967; 113 (6): 714–715 [PubMed]
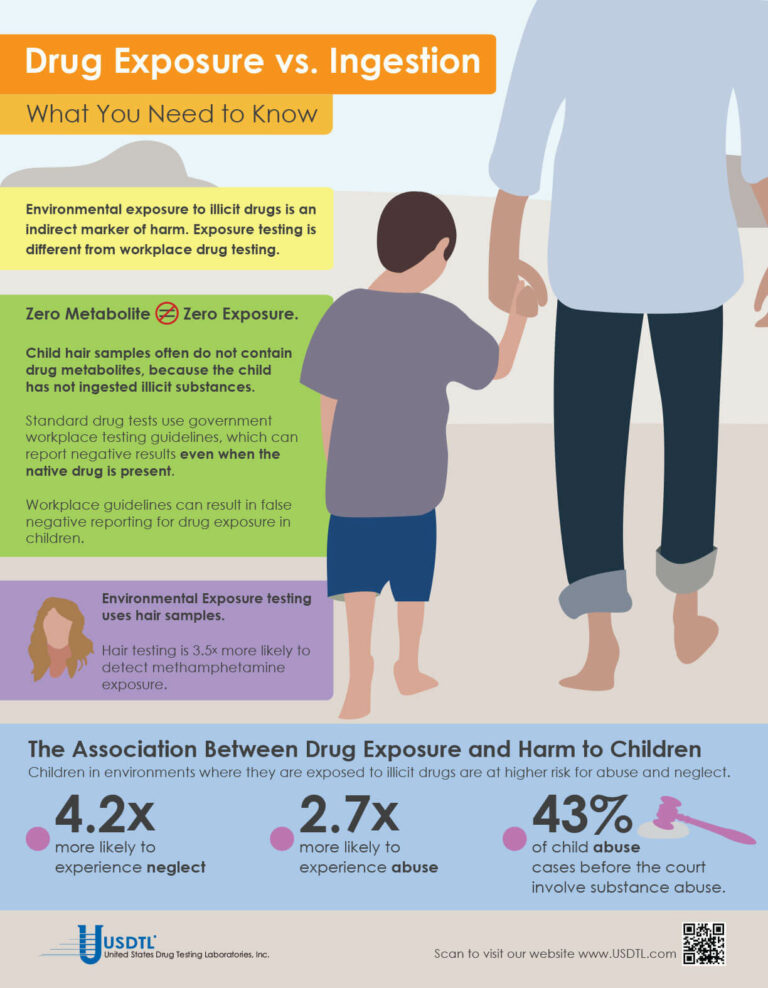
What You Need To Know: Testing for Drug Exposure vs. Ingestion
Testing for environmental exposure to illicit drugs is a powerful tool for protecting the welfare of children. Exposure testing is different from typical drug testing, and when properly done, has the potential to reduce the risk of harm to children.
No Metabolite Does NOT Mean No Exposure
Testing labs often apply government workplace testing guidelines to child exposure testing samples. Under workplace guidelines, negative results are reported when drug metabolites are absent in the testing sample, even if the native drug is present.
Child hair and nail samples for exposure testing often do not contain drug metabolites because the child has not ingested illicit substances. Adhering to workplace guidelines can result in false negative reporting for drug exposure, especially when children are involved.
Environmental Exposure
Environmental Exposure testing is most effective in alternative sample types, such as hair and fingernails. For example, hair testing is 3.5x more likely to detect methamphetamine exposure than urine testing. Typical drug testing samples are washed to remove drug biomarkers resulting from exposure. Environmental exposure testing eliminates this step.
References:
- Kelleher, K., Chaffin, M., Hollenberg, J., & Fischer, E. (1994). Alcohol and drug disorders among physically abusive and neglectful parents in a community-based sample. American Journal of Public Health, 84(10), 1586-1590.
- Murphy, J. M., Jellinek, M., Quinn, D., Smith, G., Poitrast, F. G., & Goshko, M. (1991). Substance abuse and serious child mistreatment: Prevalence, risk, and outcome in a court sample. Child abuse & neglect, 15(3), 197-211.
– Click here to download the pdf.
When a child is exposed to illegal substance abuse they often also face other coexisting obstacles to a normal life – neglect, abuse, violence, and other vulnerabilities. Substance abuse is a disease, one that often prevents adults from doing what is in a child’s best interests. Our environmental exposure test for children can help.
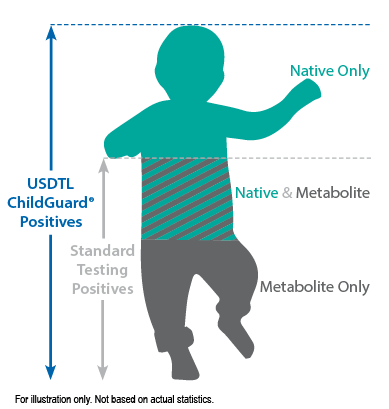
Our hair environmental exposure test is the only drug test designed to detect passive exposure to drugs and detect both native drugs and drug metabolites in the hair specimen. Drug metabolites are produced in the body only if drugs have been ingested. Children in drug exposed environments are most often not drug users themselves, so drug metabolites are typically absent in child specimens. However, the hair, like a sponge, can absorb non-metabolized drug (native drug) if it is exposed through things such as touching or being in contact with drugs or drug users.
Standard hair tests with other labs will only report a positive exposure result if drug metabolites are detected, even when the native drug is in the child’s hair specimen. Our hair environmental exposure test reports a positive result if either native drugs or drug metabolites are detected.
A hair exposure test can provide evidence of drugs in a child’s environment for the past 3 months. A positive test result suggests that the child has experienced one or more of the following: passive inhalation of drug smoke, contact with drug smoke, contact with sweat or sebum (skin oil) of a drug user, contact with the actual drug, or accidental or intentional ingestion of illegal drugs.
ChildGuard®is the only child hair test designed to detect exposure to native drugs and drug metabolites.
- The Detection of Delta-9-tetrahydrocannabinol, Delta-8-tetrahydrocannabinol, Delta-10-tetrahydrocannabinol, and Cannabidiol in Hair Specimens
- Umbilical Cord Tissue Testing for Ketamine
- Drugs of Abuse: A DEA Resource Guide (2024)
- Beyond THC and CBD: Understanding New Cannabinoids
- New Xylazine, Psilocin, Gabapentin, Dextromethorphan, and Extended Cannabinoids Testing at USDTL
- Psilocin: The Magic Behind the Mushroom
- Fetal Fentanyl Syndrome: Why Detecting Newborn Fentanyl Exposure Matters Now More Than Ever
- DMT: An Overview
- October 2025 (1)
- July 2025 (3)
- May 2025 (2)
- April 2025 (2)
- March 2025 (2)
- February 2025 (1)