Gabapentin Abuse
Showing: umbilical cord testing

There are many variables regarding the analyses of substance abuse testing. Clients will often ask about specifics pertaining to the determination of time, dose and frequency when detecting substance(s) of abuse.
When testing a reservoir matrix- a material or substance which can accumulate and retain drug and alcohol biomarkers (eg., urine, blood, hair, nail, umbilical cord, or meconium, etc.), the reported quantitation of a drug or its metabolite cannot be used to determine when/if a specific substance was used, how much of a substance was used or how often a substance was used. Test results show only if a substance was detected or not detected.
A specimen’s window of detection provides an estimated timeframe for detecting substance(s) of abuse. Based on extensive research studies, the generally accepted windows of detection for specimens used in our testing are as follows:
- Scalp Hair- Up to approximately 3 months prior to collection.
- Fingernail- Up to approximately 3-6 months prior to collection.
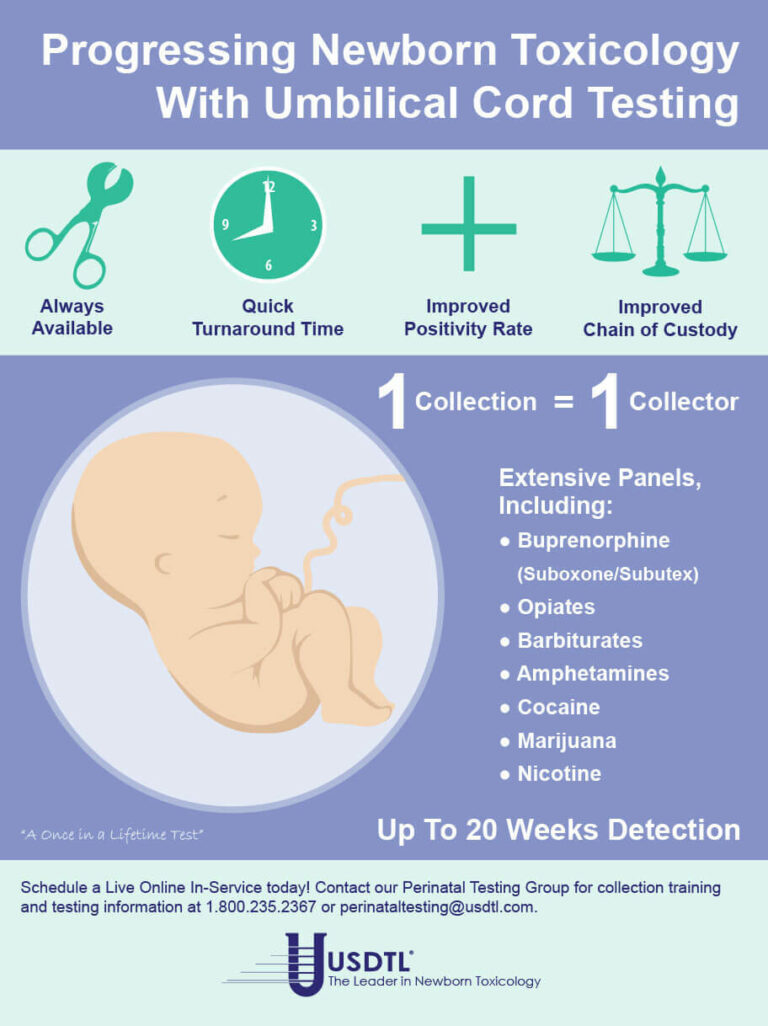
- Umbilical Cord- Up to approximately 20 weeks prior to birth.
- Meconium- Up to approximately 20 weeks prior to birth.
- Urine- Up to approximately 2-3 days prior to collection.
- Blood (PEth)-May be up to approximately 2-4 weeks prior to collection.
It is important to know that the interpretation of drug testing results may be determined by a Medical Review Officer (MRO). A Medical Review Officer is a licensed physician (MD or DO) who has knowledge of substance abuse disorders and has the appropriate medical training to interpret and evaluate an individual’s positive test result together with his or her medical history and any other relevant biomedical information.1This is an incredibly important aspect of drug testing. A laboratory can detect substances, but an MRO may be used to interpret what that detection means.
1. Journal of Occupational and Environmental Medicine: (January 2003-Volume 45-Issue 1-p 102-103) Qualifications of Medical Review Officers (MRO’s) in Regulated and Nonregulated Drug Testing. Departments: ACOEM Consensus Opinion Statement
What you need to know about meconium collection.
by Michelle Lach, MSIMC
Meconium is the first stool of a newborn infant. It is produced in utero and consists of materials such as epithelial cells, bile, mucous, and more. In most newborns, meconium is generally passed in the first day or so of life, has no odor, and appears as a very dark, tar-like substance. This helps distinguish meconium from the next phase of passage called transitional stool.
Transitional stool will start to have an odor and present with a more brown, green, or yellow color as the newborn starts digesting milk. When drug testing the meconium of a newborn, it is important to note this difference since only meconium is created during gestation and transitional stool is created after birth. Collection of any stool other than meconium for drug testing purposes may result in a rejected specimen.
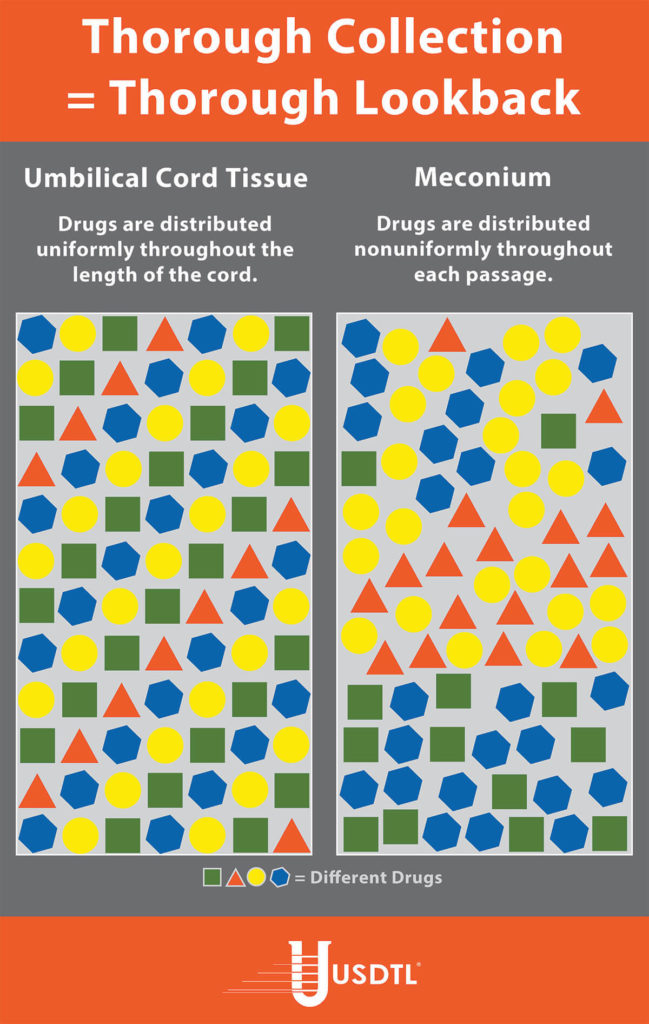
Unlike umbilical cord tissue, drugs are not distributed uniformly throughout the meconium specimen (see Figure 1). Because of this, the collection of the entire mass of meconium is highly encouraged to assure that there will be enough specimen to test, and that the maximum window of drug detection is achieved. It can take multiple passages of meconium before the newborn begins the transitional stool phase.
We require a minimum of 3 grams of meconium to be able to properly run our tests, so collecting the entire passage of meconium from newborns that have been exposed to substances of abuse is highly critical since they tend to have lower birth weights and create less specimen in the first place. If there is not enough specimen to run the test, the results are reported out as QNS. Quantity Not Sufficient (QNS) is a result of not having a sufficient quantity (volume) of specimen to test for the panels ordered.
Neonatal Drug Withdrawal
By Freepik© Studio
Maternal Nonnarcotic Drugs that cause neonatal psychomotor behavior consistent with withdrawal.
The Onset of Newborn Withdrawal Symptoms is Highly Variable
| Drug | Onset of Signs |
| Diazepam | Hours to Weeks |
| Alcohol | 3-12 Hours |
| Heroin | 24 Hours |
| Sedatives | 1-3 Days |
| Methadone | 1-7 Days |
| Opiates | 1-7 Days |
| Barbiturates | 1-14 Days |
– Click here to download the pdf.
Read an excerpt from the article Neonatal Drug Withdrawal below:
Signs characteristic of neonatal withdrawal have been attributed to intrauterine exposure to a variety of drugs. Other drugs cause signs in neonates because of acute toxicity. Chronic in utero exposure to a drug (eg, alcohol) can lead to permanent phenotypical and/or neurodevelopmental-behavioral abnormalities consistent with drug effect. Signs and symptoms of withdrawal worsen as drug levels decrease, whereas signs and symptoms of acute toxicity abate with drug elimination. Clinically important neonatal withdrawal most commonly results from intrauterine opioid exposure. The constellation of clinical findings associated with opioid withdrawal has been termed the neonatal abstinence syndrome (NAS). Among neonates exposed to opioids in utero, withdrawal signs will develop in 55% to 94%.1,2 Neonatal withdrawal signs have also been described in infants exposed antenatally to benzodiazepines,3,4 barbiturates,5,6 and alcohol.7,8
— Neonatal Drug Withdrawal https://doi.org/10.1542/peds.2011-3212
References:
-
Harper RG, Solish GI, Purow HM, Sang E, Panepinto WC. The effect of a methadone treatment program upon pregnant heroin addicts and their newborn infants. Pediatrics. 1974 ; 54 (3): 300–305 [PubMed]
-
Ostrea EM, Chavez CJ, Strauss ME. A study of factors that influence the severity of neonatal narcotic withdrawal. J Pediatr. 1976; 88 (4 pt 1): 642–645 [PubMed]
-
Rementería JL, Bhatt K. Withdrawal symptoms in neonates from intrauterine exposure to diazepam. J Pediatr. 1977; 90 (1): 123–126 [PubMed]
-
Athinarayanan P, Piero SH, Nigam SK, Glass L. Chloriazepoxide withdrawal in the neonate. Am J Obstet Gynecol. 1976; 124 (2): 212–213 [PubMed]
-
Bleyer WA, Marshall RE. Barbiturate withdrawal syndrome in a passively addicted infant. JAMA. 1972; 221 (2): 185–186 [PubMed]
- Desmond MM, Schwanecke RP, Wilson GS, Yasunaga S, Burgdorff I. Maternal barbiturate utilization and neonatal withdrawal symptomatology. J Pediatr. 1972; 80 (2): 190–197 [PubMed]
- Pierog S, Chandavasu O, Wexler I. Withdrawal symptoms in infants with the fetal alcohol syndrome. J Pediatr. 1977; 90 (4): 630–633 [PubMed]
- Nichols MM. Acute alcohol withdrawal syndrome in a newborn. Am J Dis Child. 1967; 113 (6): 714–715 [PubMed]
Dear Valued Client,
We are proud to announce that we are the first laboratory in the world to be ISO/IEC 17025 accredited for drug and alcohol testing in umbilical cord, fingernail, and toenail specimens. On September 4, 2015, USDTL attained ISO/IEC 17025 accreditation showing full compliance with the international testing standards. We have received our accreditation from ANSI-ASQ National Accreditation Board, demonstrating technical competence in the field of forensic testing. The scope of our ISO/IEC 17025 accreditation encompasses all specimen types and methods of analysis utilized in our laboratory.
Our laboratory has always maintained this level of quality and competency since our humble beginnings in 1991, bringing our clients the most responsive, personal service in the drug and alcohol testing industry. ISO/IEC 17025 accreditation reaffirms that commitment to our clients, for all aspects of our testing and client advocacy. You can always have absolute confidence that the results of every specimen tested by our laboratory will meet the highest of international standards.
ISO/IEC 17025 is the single most important standard applied to testing and calibration laboratories around the globe. Laboratories accredited to the ISO/IEC 17025 standard have demonstrated that they are technically competent and able to reproducibly generate accurate, precise and consistent data.
The practical benefits for clients of USDTL of ISO/IEC 17025 accreditation are seen on a continual basis:
- Continuously produced testing results of the highest quality, validity, and integrity;
- Improved customer communication and resolution of customer issues;
- Continual improvement of our management system, with an emphasis on the responsibilities of senior management;
- Fast resolution of laboratory issues regarding methods and equipment.
- Evidential acceptance of USDTL laboratory results in virtually all jurisdictions.
To view our certificate of accreditation by ANAB 17025:2017 Forensic Science Testing and Calibration Lab. If you have questions about ISO/IEC 17025 accreditation, please contact us at clientservices@usdtl.com or 800.235.2367.
Sincerely,
Adam Negrusz, Ph.D., F-ABFT
Laboratory Director

A: Morphine is the predominant metabolite of heroin, but morphine is also a stand alone drug and a metabolite of codeine. Some mothers are provided morphine during delivery. Historically, there have been instances where heroin using moms could not be distinguished from moms given morphine during delivery. Meconin is a contaminating constituent from poppy that is present in heroin. Therefore, like Monoacetylmorphine – a metabolite of heroin, the presence of Meconin indicates the use of heroin and when found in newborn specimens indicate fetal exposure to heroin.
USDTL offers Meconin testing in both umbilical cord tissue and meconium.
Click here to find out more about our testing services
Bob Demaree, Clinical Projects Manager at USDTL is at The Fetus & Newborn: State-of-the-Art Care conference in Las Vegas, NV this week. He welcomes every attendee to stop by and learn more about our newborn testing. More importantly our newest assay which can measure an alcohol biomarker in umbilical cord tissue.
Fetal alcohol exposure has been recognized as the leading cause of preventable mental retardation and birth defects. Each year in the United States approximately 40,000 alcohol exposed newborns are diagnosed with Fetal Alcohol Spectrum Disorder. This condition can result in a variety of physical, behavioral and learning disorders. A recent SAMHSA survey reviewing data collected in 2009-2010 reported that 4.7 percent of pregnant women admitted binge or heavy drinking. Early identification is a key factor to improving outcomes for this group. Currently, identification relies on maternal
self-report or the presence of a set of unique physical characteristics. Self-report has limited value in determining alcohol exposure and the physical characteristics may not appear until later in child’s development.
A positive result from our CordStat EtOH is an indication of risky alcohol drinking behavior in the last two to four weeks of pregnancy.
To find out more call customer serivce at 800.235.2367.
Q: How long do I have to dispute a result?
A: USDTL saves negative specimens for seven days after initial accessioning. Seven days is longer than the customary three days that most labs retain negatives. This duration should allow clients a reasonable time to decide if dispute resolution is needed, to contact customer service and initiate the process. Positive specimens are stored frozen for one year following accessioning. Our Client Services Representatives will provide you with the necessary paperwork for you to sign and return to initiate the re-test process. Once the paperwork is in order, Client Services will return a re-test result to you in one to two working days. If you have any questions after receiving the results, please contact Client Services and they will either assist you or direct you to one of our forensic toxicologists to discuss the case with you.
- USDTL’s Integration and Partnership With CourtFact
- New Year, New Capabilities: Offering Forensic & Clinical Testing Options
- Weather Delay
- The Detection of Delta-9-tetrahydrocannabinol, Delta-8-tetrahydrocannabinol, Delta-10-tetrahydrocannabinol, and Cannabidiol in Hair Specimens
- Umbilical Cord Tissue Testing for Ketamine
- Drugs of Abuse: A DEA Resource Guide (2024)
- Beyond THC and CBD: Understanding New Cannabinoids
- New Xylazine, Psilocin, Gabapentin, Dextromethorphan, and Extended Cannabinoids Testing at USDTL
- January 2026 (3)
- October 2025 (1)
- July 2025 (3)
- May 2025 (2)
- April 2025 (2)
- March 2025 (2)