Maintaining Chain of Custody Protects Health Institutions and Newborns
Showing: drug testing

By Joseph Salerno
The movement and location of physical evidence from the time it is obtained until the time it is presented in court is the legal definition of chain of custody. The results of any newborn alcohol or substance of abuse test performed at USDTL may eventually be presented as evidence in a court of law, and this is why USDTL maintains universal chain of custody regardless of the client source of testing specimens. A court can exclude the results of a test if a chain of custody for the newborn sample was not maintained by the hospital and USDTL.
Chain of custody for specimens sent to USDTL is maintained as a chronological paper trail of collection and transfers of specimens throughout the testing process. The paper trail is signed and dated by each person who handles the specimen, both when they receive the specimen into their own hands, and when they hand it off to the next person in the process. Less transfers of a specimen that need to be documented is better for the chain of custody overall. A well maintained and legal chain of custody begins at the time of specimen collection and continues uninterrupted until test results have been presented in court, if necessary.
There are several key elements of the chain of custody for alcohol and drug test samples that must be present when samples arrive at USDTL. First, the specimen container must be sealed with an intact security seal. Next, the sample must be accompanied by a Chain of Custody and Control Form with an identification number matching the number on the specimen container. The Chain of Custody and Control Form is the first piece of the chain of custody paper trail. Thirdly, the Chain of Custody and Control Form must be signed and dated by an authorized agent from the client. If one or more of these elements are missing, USDTL must return the sample to the client.
An unbroken chain of custody ensures sample integrity in several ways that preserve the legal usefulness of alcohol and drug testing results.
Chain of custody ensures that the original sample is the same as the one that is tested and ensures that the integrity of the sample is preserved during transport. Tampering, substitution, or alteration of the sample prior to being tested is prevented by the chain of custody process, which ensures thatit has been handled only by the donor, a qualified collector, and lab testing personnel.
Maintaining chain of custody for newborn samples destined for alcohol and drug testing is a simple process, but all those who handle a drug testing specimen need to be vigilant about the process nonetheless. Diligent maintenance of chain of custody is always in the child’s best interest. Unfortunately, it is only when the legal impact of an improperly maintained chain of custody is realized, that the full value of a well maintained chain of custody is understood. Ultimately, chain of custody protects the institution that is collecting the specimen, as well as the newborn whose health and well-being may rely on the results of a USDTL alcohol or drug test.
Reference: Giannelli, P. (1996). Forensic Science: Chain of Custody. Criminal Law Bulletin, 32(5), 447-465.
Please click here to read the full article by Eric Frazer, Ph. D., and Linda Smith, Ph. D., in our Fall issue of Substance.
One of the most common issues that arises in Juvenile and Family Court is parental substance abuse. Once this allegation has been raised, there is immediate concern about the child’s safety and well-being. In particular, there is often concern about neglect and abuse. For example, will the parent prioritize drug seeking over caring for the child? Will the parent drive under the influence, with the child in the vehicle? Less commonly mentioned in the courtroom, especially in the family courtroom, is potential child exposure to drugs. Unfortunately, this is a significant risk to children, and should be considered and discussed in every case.
One of the challenges family lawyers and courts face is how to properly investigate the substance abuse allegation and determine if it is a valid concern. Gathering and organizing the most relevant information has historically been difficult to do because of a lack of awareness regarding what is most relevant and important. Fortunately, the drug testing lab can bridge that gap of uncertainty, especially when there is an allegation about child exposure.
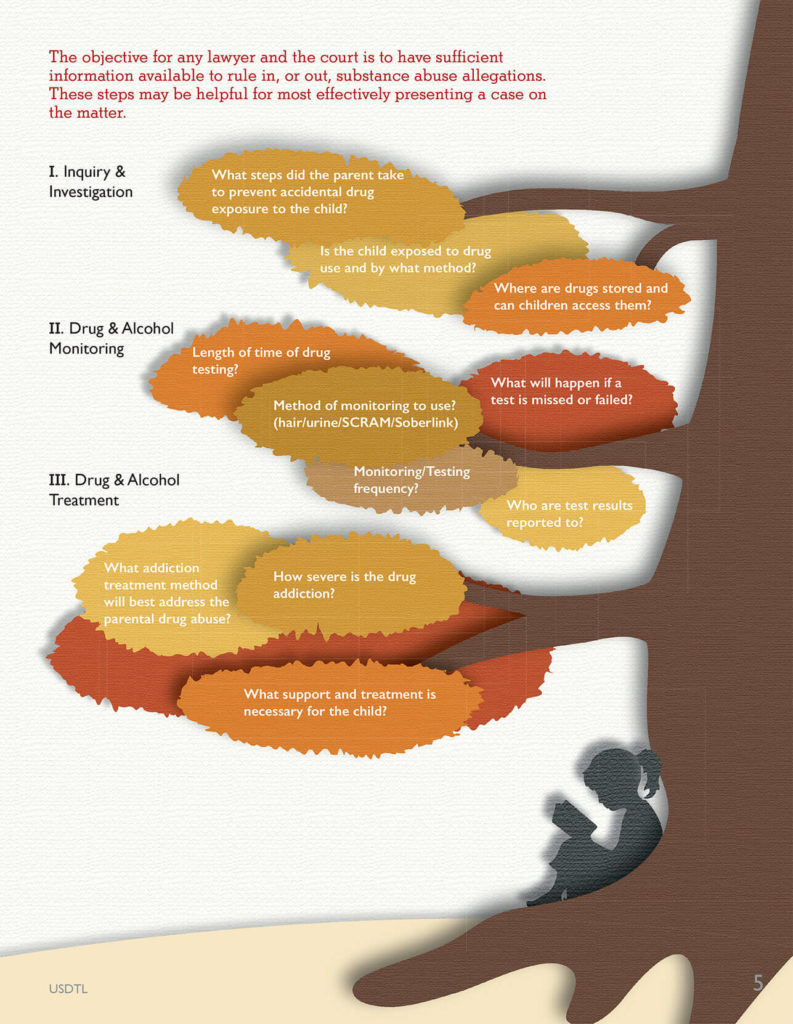
The objective for any lawyer and the court is to have sufficient information available to rule in, or out, the substance abuse allegations. This information may be presented via admissible evidence, witness testimony, and/or expert testimony. The following pointers may be helpful to legal professionals so that they are able to most effectively present a case on this matter.
Step 1 – Inquiry & Investigation
One of the first steps in evaluating a substance abuse allegation is to ask research informed questions so that the most relevant data can be gathered. Questions should be focused on potential exposure of the children to parental drug possession or use. For example, relevant questions may include:
- Where does the defendant allegedly store the drugs?
- Does the child have easy access to these storage containers and locations?
- Is the child typically present when the drugs are used?
- In what ways may the child have been exposed?
- What steps, if any, did the parent take to protect the child from accidental exposure?
Step 2 – Drug & Alcohol Monitoring
If a parent tests positive on an alcohol or drug test, this may result in a motion, agreement, or court order for ongoing monitoring. However, many questions then arise. For example, how long should the monitoring extend? Should it
include both alcohol and drugs? What type of drug monitoring method should be used (urine/hair/SCRAM/Soberlink, etc.)?
Dear Valued Client,
We are proud to announce that we are the first laboratory in the world to be ISO/IEC 17025 accredited for drug and alcohol testing in umbilical cord, fingernail, and toenail specimens. On September 4, 2015, USDTL attained ISO/IEC 17025 accreditation showing full compliance with the international testing standards. We have received our accreditation from ANSI-ASQ National Accreditation Board, demonstrating technical competence in the field of forensic testing. The scope of our ISO/IEC 17025 accreditation encompasses all specimen types and methods of analysis utilized in our laboratory.
Our laboratory has always maintained this level of quality and competency since our humble beginnings in 1991, bringing our clients the most responsive, personal service in the drug and alcohol testing industry. ISO/IEC 17025 accreditation reaffirms that commitment to our clients, for all aspects of our testing and client advocacy. You can always have absolute confidence that the results of every specimen tested by our laboratory will meet the highest of international standards.
ISO/IEC 17025 is the single most important standard applied to testing and calibration laboratories around the globe. Laboratories accredited to the ISO/IEC 17025 standard have demonstrated that they are technically competent and able to reproducibly generate accurate, precise and consistent data.
The practical benefits for clients of USDTL of ISO/IEC 17025 accreditation are seen on a continual basis:
- Continuously produced testing results of the highest quality, validity, and integrity;
- Improved customer communication and resolution of customer issues;
- Continual improvement of our management system, with an emphasis on the responsibilities of senior management;
- Fast resolution of laboratory issues regarding methods and equipment.
- Evidential acceptance of USDTL laboratory results in virtually all jurisdictions.
To view our certificate of accreditation by ANAB 17025:2017 Forensic Science Testing and Calibration Lab. If you have questions about ISO/IEC 17025 accreditation, please contact us at clientservices@usdtl.com or 800.235.2367.
Sincerely,
Adam Negrusz, Ph.D., F-ABFT
Laboratory Director

 On August 18, 2014, the pain reliever tramadol (Ultram®) will be scheduled as a Class IV controlled substance by the U.S. Drug Enforcement Administration.1 Tramadol is a synthetic, opioid pain reliever similar in strength to codeine. In 2012, more than 40 million tramadol prescriptions were written in the United States, more than any other opioid medications except for hydrocodone and oxycodone.2
On August 18, 2014, the pain reliever tramadol (Ultram®) will be scheduled as a Class IV controlled substance by the U.S. Drug Enforcement Administration.1 Tramadol is a synthetic, opioid pain reliever similar in strength to codeine. In 2012, more than 40 million tramadol prescriptions were written in the United States, more than any other opioid medications except for hydrocodone and oxycodone.2
In 1994, clinical data suggested tramadol had a very low abuse potential.3 Research has since shown that tramadol can produce a euphoric high similar to oxycodone. At doses greater than prescription levels, tramadol has addiction reinforcing effects similar to morphine and oxycodone.
Between 2004-2012, emergency department visits related to illicit tramadol use increased from 4,800 incidents to more than 16,000.1 Adverse effects of tramadol include sedation, dizziness, respiratory depression, seizures, apnea, and death.4
Withdrawal symptoms often occur following both abrupt and tapered discontinuation of tramadol use, suggesting an issue of drug dependence for tramadol users.
In the year 2000, tramadol was present in only 82 law enforcement drug seizures. This number increased to 1806 by 2012.1 A 2002 study found that 87 out of 140 healthcare professionals testing positive for tramadol use obtained the drug with illegal prescriptions.
Tramadol is available in both single dose (25-100 mg) and extended release (100-300 mg) forms. Tramadol abusers can crush extended release tablets and ingest them for an instant, high-dose euphoria similar to OxyContin but without the cognitive impairment.2
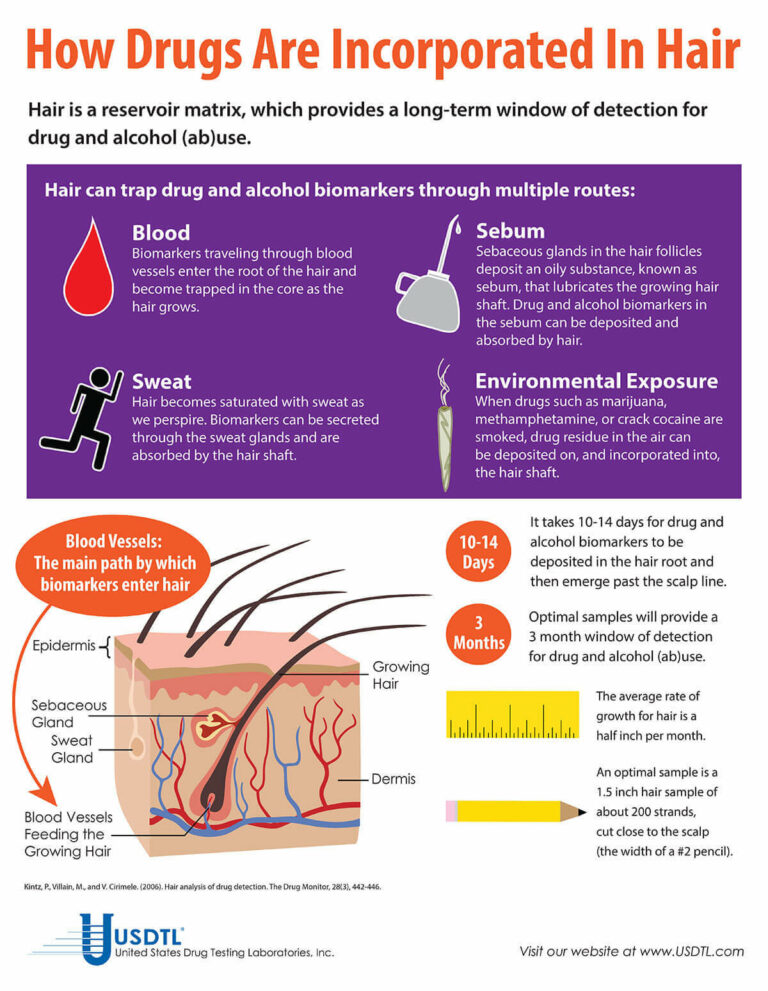
Fingernail testing detects tramadol use for up to six months, while hair provides a three month look-back. Tramadol testing is available in USDTL’s extended drug panels. If you have questions or need more information about tramadol testing, contact our Client Services group at 800•235•2367 or at clientservices@usdtl.com.
References
1. Office of Diversion Control, Drug and Chemical Evaluation Section. (May 2013). Schedules of Controlled Substances: Placement of Tramadol into Schedule IV. Retrieved from https://www.dea.gov/drug-information/drug-scheduling
2. Nadia Awad. (2014). Now What? DEA Tosses Tramadol in Schedule IV. Retrieved from http://www.medpagetoday.com
3. John Fauber. (2013). Killing Pain: Tramadol the “Safe” Drug of Abuse. Retrieved from http://www.medpagetoday.com
4. Senay, E.C., Adams, E.H., et al. (2003). Physical Dependence on Ultram (tramadol hydrochloride): Both opioid-like and atypical withdrawal symptoms occur. Drug and Alcohol Dependence, 69:233-241. DOI: 10.1016/s0376-8716(02)00321-6
- Drug Classes and Neurotransmitters: Amphetamine, Cocaine, and Hallucinogens
- Environmental Exposure Testing for Delta-8 THC, Delta-9 THC, Delta-10 THC, and CBD
- Bromazolam and Synthetic Benzodiazepines
- Winter Weather Delay Update
- Tianeptine
- Revolutionizing DUI Interventions: Wisconsin’s Breakthrough in Biomarker Testing for Impaired Drivers
- 3 FAQs You Should Know About Newborn Drug Testing
- The Brain Chemistry Behind Tolerance and Withdrawal
- March 2024 (1)
- February 2024 (1)
- January 2024 (3)
- December 2023 (1)
- November 2023 (1)
- August 2023 (1)