USDTL Serves Its First Client using the new CordStatEtOH
Showing: umbilical cord testing
Two days after the press release announcing our new CordStat EtOH assay, USDTL serves it’s first client testing umbilical cord for alcohol biomarkers. Welcome CordStat EtOH customers:
.jpg)
Specimen custodian, Senovia pulled the sample

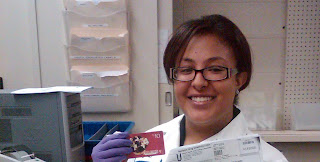
Justin ran the first CordStat EtOH batch
.jpg)
Kyla accessioned the specimen.
.jpg)
Rosie reviewed and posted the result.

- Drug Classes and Neurotransmitters: Amphetamine, Cocaine, and Hallucinogens
- Environmental Exposure Testing for Delta-8 THC, Delta-9 THC, Delta-10 THC, and CBD
- Bromazolam and Synthetic Benzodiazepines
- Winter Weather Delay Update
- Tianeptine
- Revolutionizing DUI Interventions: Wisconsin’s Breakthrough in Biomarker Testing for Impaired Drivers
- 3 FAQs You Should Know About Newborn Drug Testing
- The Brain Chemistry Behind Tolerance and Withdrawal
- March 2024 (1)
- February 2024 (1)
- January 2024 (3)
- December 2023 (1)
- November 2023 (1)
- August 2023 (1)



.jpg)

.jpg)
