White Papers
Ethyl Glucuronide (EtG) and Ethyl Sulfate (EtS) Concentrations Following Use of Ethanol Containing Mouthwash
USDTL Research
By: Joseph T. Jones, Mary R. Jones, Charles A. Plate, Douglas Lewis
Published: November 30, 2006

Mouthwash and other oral hygiene. | Sourced by Freepik©
Introduction
Ethyl glucuronide (EtG) and ethyl sulfate (EtS) are minor nonoxidative direct biomarkers of ethanol ingestion. The 2-5 day detection window of EtG and EtS is superior to direct ethanol measurements, which is approximately 1 hour per standard drink. The sensitivity of EtG and EtS in urine for beverage ethanol consumption is greater than 90 percent and is therefore an extremely important assay for the early detection of relapse in alcohol abstention programs. Other indirect markers, such as CDT, GGT, MCV, EDAC, WBAA, and FAEE have sensitivities of less than 90 percent and are therefore useless as a key indicator of relapse.
The controversy outlined in the Substance Abuse Treatment Advisory raises the issue of the detection of EtG and EtS in urine as being too sensitive. To date, there is little if any published data on the correlation of unintentional ethanol exposure and the detection of EtG and EtS in urine.
One possible source of unintentional ethanol ingestion is the use of ethanol-containing mouthwashes and breath sprays. These products can contain between 8-26% ethanol by weight. A typical 32-fluid oz package of ethanol-containing mouthwash can contain up to 20 standard 12g drinks. Ethanol is easily absorbed in the oral cavity, and inevitably, some will trickle down the back of the throat.
Experimental
The use of mouthwash was studied by using Target Brand Antiseptic Mouthrinse –Spring Mint (ethanol 21.6%) as described on the package directions, which indicates a 20 mL dose (3/4 capful) swished between the teeth for 30 seconds. After both subjects demonstrated negative EtG and EtS baseline results, this procedure was initiated and carried out once an hour for eight hours. Urine specimens were collected at 2 hours, 4 hours, 6 hours, 8 hours, and 16 hours (first void the next morning). The specimens were analyzed at USDTL for creatinine and ethanol on an Olympus AU640 using standard protocols. The limit of detection for urine ethanol was 3 mg/dL. EtG and EtS determinations were performed at USDTL using standard protocols on an API 2000 LCMSMS. The limit of detection for EtG and EtS was 38.7 ng/mL and 7.2 ng/mL, respectively.
Study Subjects
The two subjects that volunteered for this study are employees of USDTL. Subject A was a 64 kg 44 year old white female. Subject B was a 127 kg 41 year old white male. Both subjects are self reported social drinkers who abstained from social drinking during study segments.
Results
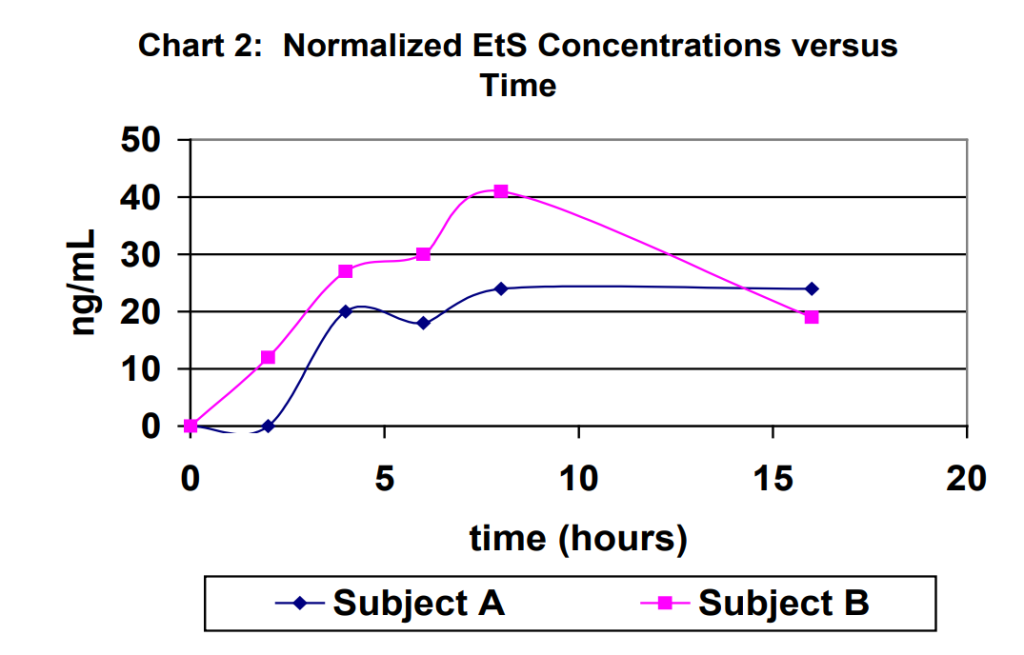
Urine specimens for each subject were captured at 2 hours, 4 hours, 6 hours, 8 hours and 16 hours (first void next morning). The results obtained for the urine specimens are outlined in Table 1. For purposes of comparison, the results were normalized to a creatinine level of 100 mg/dL in Table 1. Normalized EtG results are shown in Chart 1. Normalized EtS results are shown in Chart 2.

Discussion
Ethyl glucuronide and ethyl sulfate are very sensitive direct biomarkers of ethanol intake, both intentional and unintentional. This study demonstrated that it is possible to generate detectable concentrations of EtG and EtS by using ethanol-containing mouthwash as directed on the manufacturer’s label with as few as 2-3 rinses. The highest EtG and EtS concentration achieved using the ethanol containing mouthwash an excessive number of times over an eight-hour period of time was 366 ng/mL and 73 ng/mL, respectively.
The data generated for this study was comparable to recently published data.5 The published study listed a maximum EtG concentration of 345 ng/mL for one individual ninety minutes after using 4 ounces of Cepacol (ethanol 12%) over a 15 minute period. Our study generated a maximum of 366 ng/mL after 6 hourly doses of Spring Mint (ethanol 21.6%).
In conclusion, it is imperative that individuals participating in a program that requires ethanol-abstinence choose mouthwashes that do not contain ethanol for their personal hygiene. On the other hand, mouthwash cannot be used as an explanation for concentrations of EtG and EtS that are significantly higher than those demonstrated in this study.
Refereces
- Wurst F; Seidl S; Ladewig D; Muller-Spahn F; Alt A. Ethyl Glucuronide: on the Time Course of Excretion in Urine During Detoxification. Addiction Biology 2002, 7, 427-434.
- Helander A; Beck O. Ethyl Sulfate: a Metabolite of Ethanol in Humans and a Potential Biomarker of Acute Alcohol Intake. J Anal Tox 2006, 29, 270-274.
- Bean P. State of the Art: Contemporary Biomarkers of Alcohol Consumption. Med Lab Obs. November, 2005.
- Center for Substance Abuse Treatment. The Role of Biomarkers in the Treatment of Alcohol Use Disorders. Substance Abuse Treatment Advisory. DHHS Publication No. 06-4223. Volume 5, Issue 4, September 2006.
- Costantino A, et al. The Effect of the Use of Mouthwash on Ethylglucuronide Concentrations in Urine. J Anal Tox 2006, 30, 659-662.
Contact USDTL
1.800.235.2367
Client Services
Business Hours (CT)
Mon.-Fri. 7am-6pm
Sat. 8am-5pm
Holidays Closure
New Year's Day
Memorial Day
Independence Day
Labor Day
Thanksgiving Day
Christmas Day
Request Your Collection Supplies
For your convenience, USDTL provides test collection supplies at no additional charge.
Newsletters, Posters, and Catalogs
Our print materials will keep you up to date on the latest news in drug and alcohol testing.