Blog

ToxTime: Ethyl Glucuronide (EtG) in Hair and Nail – A review and Recent Updates
Please join us for ToxTime with Joseph Jones, Ph.D., NRCC-TC, Chief Operating Officer at USDTL, as he presents on what is new with using hair and nail to detect Ethyl Glucuronide (EtG), a long-term ethanol biomarker.
What You’ll Learn
- What is Ethyl Glucuronide (EtG)
- Ethyl Glucuronide (EtG) testing in
- Urine
- Hair
- Nail
- Umbilical Cord Tissue
- A brief overview of previous studies
- And more…
Click Here to Watch the Webinar
When drugs are swallowed, they may be broken down (metabolized) by enzymes and/or absorbed using transporters in cells found in the small intestine. Grapefruit juice can cause problems with these enzymes and transporters, causing too much or too little drug in the body.
Some drugs, like statins used to lower cholesterol, are broken down by enzymes. Grapefruit juice can block the action of these enzymes, increasing the amount of drug in the body and may cause more side effects.
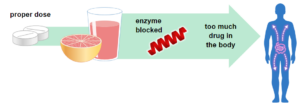
Photo from the U.S. Food & Drug Administration
Other drugs, like Allegra (fexofenadine) used to treat allergies, are moved by transporters into the body’s cells. Grapefruit juice can block the action of transporters, decreasing the amount of drug in the body and may cause the drug to not work as well.
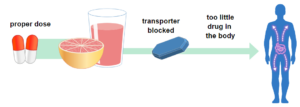
Photo from the U.S. Food & Drug Administration
Grapefruits: Food and Drug Interaction
The Canadian Medical Association Journal (CMAJ) released a review of drugs that interact with Grapefruit, “43 drugs in dangerous ways.”
List of medications that may cause severe side-effects when interacting with grapefruit.

Image by CBC Radio Canada, Information from the Canadian Medical Association Journal
Information collected from:
- https://www.fda.gov/consumers/consumer-updates/grapefruit-juice-and-some-drugs-dont-mix
- https://www.cmaj.ca/content/185/4/309
- https://www.cbc.ca/news/health/grapefruit-juice-interaction-with-drugs-can-be-deadly-1.1253489
These days, having a “drink” can be deceiving. The alcohol content can vary drastically from one drink to the next.

A standard drink is any beverage that contains 14g (0.6) of pure alcohol. Amounts vary depending on the percentage of Alcohol by Volume (ABV).

Blood alcohol concentration levels for 6 drinks within 12 hours can differ greatly depending on drinking behavior and individual metabolism.

The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines binge drinking as a pattern of drinking that brings a person’s blood alcohol concentration (BAC) to 0.08 grams percent or above. This typically happens when men consume 5 or more drinks and when women consume 4 or more drinks in about 2 hours.
The Bottom Line
Self-report screening of maternal drinking during pregnancy is currently the standard test for monitoring maternal alcohol consumption and identifying alcohol-exposed newborns. However, misinformation on what defines binge drinking means self-report may not be reliable.
Alcohol biomarkers can be used as objective measurements for monitoring maternal alcohol consumption and screening for prenatal alcohol exposure.
Learn More About Alcohol Biomarker Testing
We are here to clear up some of the confusion that can surround the issue of alcohol biomarker detection and to give you the answers you need.
Contact us if you have questions or inquiries about our process or procedures. Please call 847.375.0770. Have a question but don’t want to reach out? Check out our Frequently Asked Questions (FAQs) section, where you’ll find answers to common and not-so-common questions.

Sourced by Freepik Company S.L.
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that can be used to treat depression, anxiety, or other psychological disorders. SSRIs function by blocking the reabsorption of serotonin into neurons, causing an artificially high level of serotonin in the brain. The first SSRI, fluoxetine, was introduced into the market in 1988 as a safer alternative to tricyclic antidepressants (1). From 2015-2018, 13.2% of adults used antidepressants, and SSRIs account for approximately 70% of antidepressants prescribed (2,3).
Approximately 5.5% of pregnant women in North America used SSRIs during their pregnancy in 2019 (4). The medical opinion on the safety of taking SSRIs during pregnancy is complex. Untreated mental illness of an expecting mother can be a threat to the developing fetus, with an increased risk of preterm birth and low birth weight (5). Additionally, the babies of untreated depressed mothers can have been exposed to higher levels of cortisol, which increases the baby’s risk of developing mental illness later in life.
However, SSRI use during pregnancy might pose a risk to the developing fetus since about 30 percent of babies exposed in-utero will experience neonatal abstinence syndrome upon birth (5). Additionally, some studies have suggested SSRI use during pregnancy can be linked to an increased risk of persistent pulmonary hypertension, which is a birth defect affecting the baby’s lungs. Further studies have linked using a specific SSRI, paroxetine, during pregnancy to cardiac, brain, and abdominal birth defects (6). Still, other studies draw no link between SSRI use during pregnancy and adverse fetal outcomes. Overall, the various studies on SSRIs and their safety during pregnancy are conflicting, and more data is needed to draw more reliable conclusions.
Resources:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC181155/
- https://www.cdc.gov/nchs/products/databriefs/db377.htm
- https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00035/full
- https://www.sciencedirect.com/science/article/abs/pii/S0165032719331854
- https://www.hopkinsmedicine.org/health/wellness-and-prevention/antidepressants-and-pregnancy-tips-from-an-expert
- https://www.cdc.gov/pregnancy/meds/treatingfortwo/features/ssrisandbirthdefects.html
Sign up here to learn more and receive our newsletter on the latest updates with USDTL.
We are excited to inform you that we have extended our environmental exposure testing to include 3 new panels. This means we are now testing for more substances for exposure. View our panels here!
Our testing will now include detection of:
- Fentanyl
- Meperidine
- Tramadol
If you have questions you can contact your account manager or speak to one of our representatives by calling 800.235.2367 or by emailing us here.
To learn more, watch our educational ToxTime event Environmental Exposure Drug Testing 101 ChildGuard.

Newborn Toxicology – A Review and Recent Developments
Please join us for ToxTime with Joseph Jones, Ph.D., NRCC-TC, Chief Operating Officer at USDTL, as he discusses the evolution of drug testing in various newborn specimen types, followed by a brief Q&A.
What You’ll Learn
- The pros and cons of newborn specimen types
- The evolution of newborn testing from urine to meconium to umbilical cord tissue
- A brief overview of previous studies
- And more…
Click Here to Watch the Webinar.
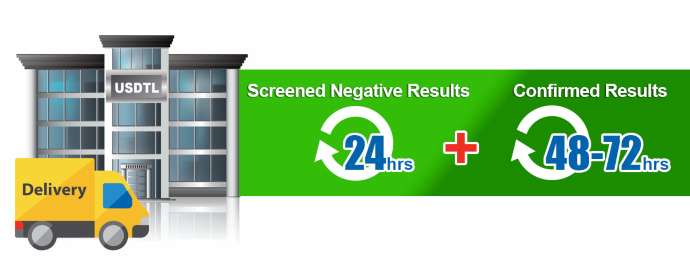 Generally, the standard turnaround time for reporting negative screening test results is the next business day, with an additional 1-2 business days for specimens that require confirmatory testing. Turnaround time begins from receipt of the valid specimen –accompanied by a properly documented valid order– into the laboratory. Some tests require additional time to process and will fall outside the standard turnaround time window.
Generally, the standard turnaround time for reporting negative screening test results is the next business day, with an additional 1-2 business days for specimens that require confirmatory testing. Turnaround time begins from receipt of the valid specimen –accompanied by a properly documented valid order– into the laboratory. Some tests require additional time to process and will fall outside the standard turnaround time window.
As an accredited laboratory, we have an extensive list of criteria that must be met to ensure each specimen result is forensically defensible evidence. Given the accreditation guidelines, reanalysis of evidence may be needed on specimens, or specimen batches, for various reasons. Though we try our best to avoid these situations, we have protocol(s) we must follow when this happens. Unfortunately, reanalysis can shift the anticipated turnaround time for specimen results. When it is required, we ask for your understanding and patience while further testing is being performed.

The Detection of Fentanyl in Hair Specimens
Joseph Jones, Ph.D., NRCC-TC, Chief Operating Officer at USDTL discusses the toxicology of fentanyl in hair specimens followed by a brief Q&A.
As innovators in substance abuse toxicology, USDTL has been testing for fentanyl in hair specimens for over a decade, well before it was a mainstream drug of abuse. In this webinar, we share with you what we have learned about the dangers of fentanyl specifically, and the trends we have seen to help you understand why testing in hair specimens is beneficial.
What You’ll Learn
- Why fentanyl is so dangerous compared to other opioids
- Relevant data and case report overview
- Fentanyl trends in hair specimens at USDTL
- And more…
Click Here to Watch the Webinar.
- Drug Classes and Neurotransmitters: Amphetamine, Cocaine, and Hallucinogens
- Environmental Exposure Testing for Delta-8 THC, Delta-9 THC, Delta-10 THC, and CBD
- Bromazolam and Synthetic Benzodiazepines
- Winter Weather Delay Update
- Tianeptine
- Revolutionizing DUI Interventions: Wisconsin’s Breakthrough in Biomarker Testing for Impaired Drivers
- 3 FAQs You Should Know About Newborn Drug Testing
- The Brain Chemistry Behind Tolerance and Withdrawal
- March 2024 (1)
- February 2024 (1)
- January 2024 (3)
- December 2023 (1)
- November 2023 (1)
- August 2023 (1)


